– notes from a Medifem Hospital pharmacist
Sometimes at the hospital, patients reject prescribed medicines citing past undesirable experiences with it. Adverse reactions to drugs and the need to ensure drugs are dispensed and taken in a safe manner have emerged strongly as key issues in promoting patient safety in healthcare practice.
This has given birth to a distinct discipline within Pharmacy practice known as Pharmacovigilance.
Pharmacovigilance (PV or PhV), also known as drug safety, is the pharmacological science relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products. The etymological roots for the word “pharmacovigilance” are pharmakon (Greek for drug) and vigilare (Latin for to keep watch).
As a discipline that began some 170 years ago, pharmacovigilance primarily seeks to address two things;
- identify new information about hazards associated with medicines
- Prevent harm to patients.
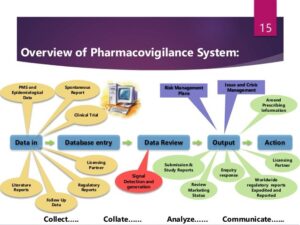
THE PROCESS & ITS OUTCOMES
To ensure that information about drugs are accurately collected for documentation, reporting and evaluation, data management is key. The steps in safety data management are;
- Data collection and verification.
- Coding of adverse reaction descriptions.
- Coding of drugs.
- Case causality assessment.
- Timely reporting to authorities
Beyond identifying new information about hazards and preventing harm, the process also seeks to;
- improve patient care and safety in relation to the use of medicines and all medical and para-medical interventions.
- drive the research of the efficacy of drugs by monitoring adverse effects right from the lab to the pharmacy and then on for many years.
- keep track of any drastic effects of drugs.
- improve public health and safety in relation to the use of medicines.
- contribute to the assessment of benefit, harm, effectiveness and risk of medicines, encouraging their safe, rational and more effective (including cost-effective) use.
- promote understanding, education and clinical training in effective communication on drugs to the public
SUB-SPECIALTIES
Pharmacovigilance is a huge and encompassing discipline but can broadly be divided into three sub-specialisms:
Operations:
This sector is where many pharmacists/drug professionals interested in drug safety jobs begin their career. Typical jobs within drug safety operations include case processor, drug safety officer/associate and drug safety manager, and of course team lead and directorships. These professionals will collect and record information during preclinical development and clinical trials, in addition to gathering real-world evidence (RWE) of adverse events reported by doctors and patients. Operations are also usually responsible for creating standard operating procedures (SOPs), individual case study reports, literature screening and regulatory expedited reporting.
Surveillance:
Professionals who focus more on surveillance tend to look towards risk management and signal detection jobs. These professionals perform analysis on the drug safety information gathered by the operations people and assist with the creation and review of aggregate reports. They also create development safety update reports (DSURs) for drugs in clinical research and periodic benefit-risk evaluation reports (PBRER) for post-market drugs. These reports ultimately help the manufacturers, scientist and Doctors to draw conclusions around the safety and efficacy of a drug.
Systems:
This division is concerned with the building and ongoing development of a fully robust and innovative system, charged with the responsibility for housing and allowing access (in various forms) to vast quantities of safety data. This safety data is usually collated by those working in operationally focused roles but is accessed by all. The systems division is constantly having to improve, and stay in line with, changing regulations and requirements for the business/ health authorities, making this a very challenging and vital aspect of drug safety roles.
INTEGRATING PhV AT MEDIFEM
In line with promoting optimum patient safety, the Pharmacy Department of the Medifem Multi-Specialist Hospital and Fertility Centre at Westlands, Accra, has as part of its broader Standard Operating Procedure, adopted certain practices to entrench Pharmacovigilance.
In the pharmacy at Medifem, documentation is one of the priorities so various classes of documents have been developed to ensure proper data-keeping. The aim is to have an ongoing robust safety data system that allows us to know the key issues and for us to be able to track specific patient concerns and report them for drug manufacturers, suppliers, doctors and other professionals in the drug-value chain.
The Adverse Drug Reaction (ADR) Book is a book dedicated to documenting all adverse reactions reported by patients. A follow up is done to get the record stated in the patient’s history to inform any future decisions by prescribers concerning the use of the medications involved in the incident.
Medications of patients on the wards are well documented and the same made readily available for cross-checking with Doctors and Nurses in the event of a reported adverse reaction.
For outpatients, prescriptions are well screened to get the diagnosis and to assess the medications in order to detect and prevent any disease-drug interaction or drug-drug interaction.
Key to our Pharmacovigilance practice here is our dedication to work with regulatory bodies, namely the Food and Drugs Authority, Ghana and manufacturers in providing data that informs statistics, trends, improvements, innovations and new initiatives. As we look to an even more enhanced drug dispensing and safety regime, continuous professional pharmacovigilance training for our pharmacy staff is a key area in focus.
 WHAT ARE THE NEW TRENDS FOR THE FUTURE?
WHAT ARE THE NEW TRENDS FOR THE FUTURE?
Outsourcing to drive operational efficiency
Specialised outsourcing in pharmacovigilance is becoming a widely used approach to coping with the growing costs of maintaining a highly qualified and trained pharmacovigilance team in-house.
Lately, more and more companies are outsourcing their pharmacovigilance tasks to achieve better regulatory compliance, higher quality, better productivity, and improved strategic decisions.
Use of Secondary Data sources that contributes to early detection of safety concerns
In the last years, the number of secondary data sources has been growing continuously and it currently includes:
- Social media;
- Clinical data and electronic health records;
- Claims files;
- Regulatory reports and filings from different areas;
Secondary data sources present unique challenges in terms of acquisition and integration with “classic” data sets. Usage of secondary data by pharmaceuticals is, as of today, just at the initial stage considering also that it is not required from the regulatory point of view.
Recently, technology providers have started offering robust and flexible platforms that help healthcare companies and manufacturers handle and integrate a wide range of file types and incorporate social media streams in their pharmacovigilance processes. Advanced algorithms and disproportionality analysis are already being expanded to include not only classical spontaneous reporting but also social media streams.
The secondary data sources together with advanced signal detection technologies help early detection of safety risks and allow to take risk minimisation measures more rapidly.
Cloud-based reporting to bring a robust global database of adverse events
Many industries are now benefiting from the ability to store and analyse huge amounts of data in the cloud. As the number of data sources grow, healthcare companies face the urgent need to optimise the intake, storage and analysis of big volumes of data.
Big Data to protect and assimilate a huge amount of information
Novel sources of real-world evidence and experimental data in digital form have become available also to pharmacovigilance professionals.
In pharmacovigilance, big data include such sources as:
- Signal detection;
- Substantiation and validation of drug or vaccine safety signals;
- Online channels and social media.
Due to its complexity, big data represent both an opportunity and a challenge. With the support of technology solutions with advanced computing capabilities, healthcare companies use big data to monitor and study drug safety in a more effective manner.
Data analytics to drive actionable insights
The effective management of safety data stored across multiple platforms is vital for a clear understanding of safety events. Manufacturing companies and healthcare professionals are striving to uncover new patterns, unknown correlations, trends, and patient preferences that can help them to ensure patients’ safety more effectively.
Nowadays, pharmacovigilance analytics provide a real opportunity to harness data effectively, ensure regulatory compliance and drive actionable insights.
As regulatory bodies especially the FDA introduce new tools to collect and evaluate adverse effects of drugs, it is imperative on practitioners to themselves institute measures within their Pharmacy/Pharmaceutical units to complement these efforts.
The writer is Pharmacist and Head of Pharmacy at the Medifem Multi-Specialist Hospital & Fertility Centre, Westlands, Accra.










